Research
Click here for FANCL's R&D Vision
Our unique approach to Mutenka Cosmetics
FANCL's own strict standards for additive-free quality.
Our unique approach to Health Food
FANCL's reliable quality to ensure that customers are able to feel secure about consuming heath foods on a daily basis.
Production
Both cosmetics and health food are manufactured under a management system in accordance with GMP standards. FANCL has its own strict standards and manufactures its products in pursuit of Upholding Quality. Our six production bases in Japan are commited to delivering safety and assurance in the products we produce for our customers.
Standards for Production Bases
- Cosmetics factories (Chiba, Shiga, Gunma) 100% GMP compliance for cosmetics
- Supplement factories (Yokohama, Mishima) 100% GMP compliance for health foods
- Food-related factories (Nagano, Mishima) 100% FSSC22000 certified
The Nagano factory/ Mishima Factory, certified by international safety standards
In November 2015, the FANCL B&H Nagano Factory received the "FSSC22000" international food safety standard certification. This sanitary factory environment for the safe production of germinated rice has been highly recognized by our business partners and the many customers who use our products. The FANCL B&H Mishima Factory also obtained the same certification in November 2022. With this certification, we will continue efforts to further enhance levels of food safety and product quality assurance, and ensure that customers in Japan and overseas can use our products with peace of mind.



Quality and safety checks
FANCL has created a management system for its quality assurance activities and established a "Cosmetic Ingredient Management Subcommittee" and "Food Ingredient Management Subcommittee" to conduct quality and safety checks.
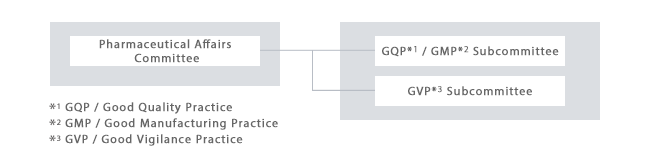
In addition, the "Pharmaceutical Affairs Committee" chaired by the President determines quality policies based on laws, and in the "GQP*1 / GMP*2 Subcommittee", "GVP*3 Subcommittee", and "Labeling and Advertising Subcommittee" under its umbrella, the optimization of the respective areas is discussed and centrally managed.
GMP Inspection
FANCL pays attention not only to the manufacturing environment of its group companies, but also to the manufacturing and quality control of the companies to which it outsources manufacturing. We regularly inspect whether contracted manufacturing companies are manufacturing in accordance with GMP standards.
GMP is a manufacturing standard established by the US FDA (Food and Drug Administration) to ensure compliance and manufacturing and quality control of cosmetics, pharmaceutical products, and food products.
Promoting Factory Automation Dedicated Production Facility for Mild Cleansing Oil
To meet the increasing demand for Mild Cleansing Oil, which was produced at three plants in Japan at a total volume of nine million bottles per year, a new dedicated production facility was built in March 2020 within the Chiba factory to consolidate production bases.
As a result, the production capacity of the filling and packaging facility is now three times faster than that of the previous machine, and the speed and automation of the production line has enabled us to reduce the number of employees involved to a quarter of what it used to be, with production capacity growing to 12 million bottles per year, an increase of more than 1.3 times.
Consolidation of production bases has reduced energy consumption, and automation of processes for heavy materials has also been achieved.
Furthermore, in September 2020, facilities that can also produce refillable containers were introduced with the aim of reducing plastic use, making the factory friendly to both the environment and people.

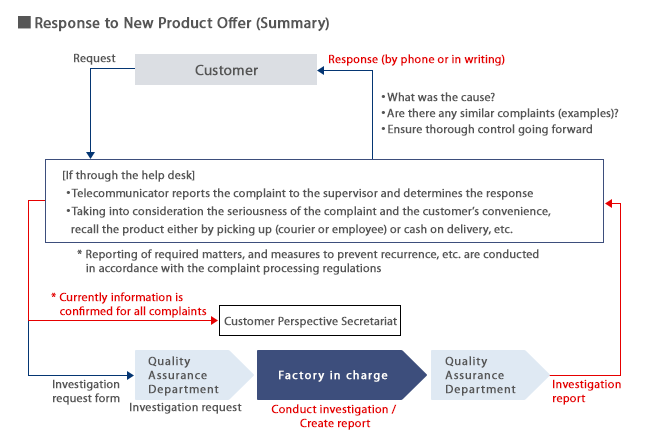
Response to Product Offer
When an inquiry is received about a product, we conduct an investigation according to the following process and respond to the customer.