Quality and Safety Assurance Framework
In order to provide customers with safe and reliable products, we have set our own strict standards for guaranteeing quality and safety in every process.
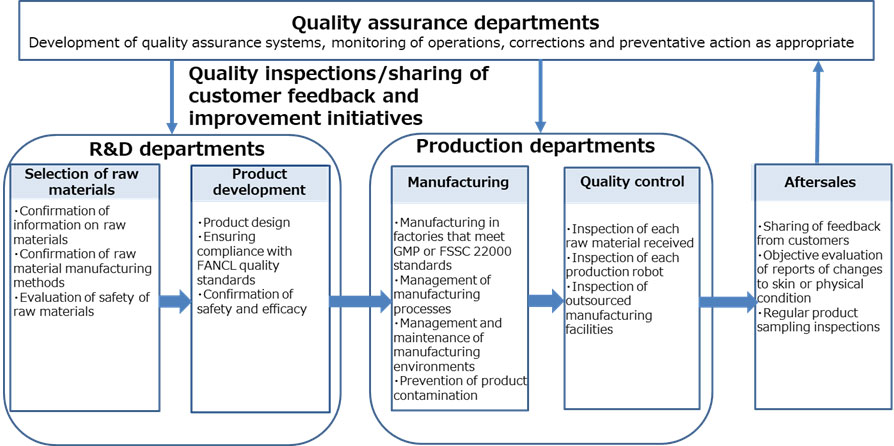
Establishing a framework for safe materials selection
When considering the use of a new raw material, our specialist departments audit it for safety, including checking information on the raw material, checking manufacturing methods, reviewing relevant literature, confirming the safety of the raw material, and inspecting for harmful substances. The decision of whether to use the raw material or not is then made by the Materials Management Working Group and the New Materials Advisory Committee, which include relevant departments, based on the results of the safety audit.
- Checking information on the raw material
Checks cover whether there are laws or regulations governing the material, its derivation and place of origin, safety data and market record, and microorganism and harmful substance inspection results data. Raw material specifications, inspection result statements, and the risk of contamination by allergens or other substances in production lines are also checked.
- Evaluating raw material quality
Inspections for microorganisms, harmful metals, allergens, residual agrochemicals, or other substances are carried out in accordance with the properties of the raw material to ensure there are no doubts about safety.
Product development in pursuit of quality and safety
Cosmetics: Product design that provides customers with naturally beautiful skin
Our Mutenka formulations do not contain any constituents that are judged to cause stress to skin so that all customers can realize their dream of naturally beautiful skin without causing damage.
- We design products that do not contain constituents with a high probability of triggering allergies (preservatives, petroleum-based surfactants, synthetic perfumes, synthetic dyes, ultraviolet stabilizers, etc.),
- We take special care with constituents that may cause skin stress, even if they also have confirmed beneficial effects on skin.
- We ensure product quality by managing every process, from planning and research through to manufacturing, in accordance with our own strict standards.
Health foods: Product design that provides consistent product quality
We ensure that our manufacturing facilities can manufacture products with consistent quality through design that includes research into formulations and manufacturing methods in a way that considers constituent weights, disintegrability, and constitutive uniformity.
- We have developed a proprietary formulation design AI program to carry out product design that minimizes the use of excipients (materials used to create a formulation)
- We focus on ease of swallowing (useability) to facilitate ongoing daily use. Decisions are made based on our own original evaluations of factors such as the thickness, roundness, and size of supplements, and the taste and smell of foods, and on home use tests.
Original quality standards to ensure safety and reliability for customers
For the products we design, we test and then verify the efficacy of a product to confirm that it can demonstrate its intended functions and carry out safety trials to confirm the use or intake of a product does not cause a change in skin or physical condition. These tests are carried out as necessary and ensure that a product fully meets FANCL quality standards.
Cosmetics
- We carry out safety verification processes (cytotoxicity tests, sensitive skin patch tests, sensitive skin usage trials, allergen tests, non-comedogenic tests, weak stimuli tests, and trials under the supervision of a dermatologist) in accordance with the product design.
Health foods
- We confirm products meet our own safety standards in areas such as contamination by harmful substances or other contaminants.
- We carry out stability trials for all products and set expiration dates accordingly. One of the indicators used in these trials is a sensory evaluation. Sensory evaluators undergo specialist training and they check required factors such as product appearance, smell, and taste over time to ensure quality up to the expiration date.
Maintenance of manufacturing environments
Aiming to build pharmaceutical-level production systems
Our factories are compliant with GMP※ and other standards. At these factories, we carry out production under manufacturing, hygiene, and quality control frameworks based on the three principles of GMP (minimize human error, prevent product contamination, and establish high-degree quality assurance systems).
- Instructional documents and records are made for each progress as part of a system to ensure traceability.
- Production is carried out in environments with air conditioning and filtration systems to build structures that ensure fine particles of foreign matter are not brought in, created, accumulated, and are expelled.
- We carry out regular employee education and quality control activities with the constant goal of realizing bottom-up practices.
Good Manufacturing Practice: Standards for production management and quality control.
Standards for production facilities
Our six cosmetics and health food manufacturing facilities in Japan engage in manufacturing in accordance with our own strict standards with the mission of providing customers with safe, reliable products that comply with various benchmarks and voluntary standards.
- Chiba factory, Shiga factory, Gunma factory: 100% GMP compliance for cosmetics
- Yokohama factory, Mishima factory: 100% GMP compliance for health foods
- Nagano factory, Mishima factory: 100% FSSC22000 certified
The Nagano factory/ Mishima Factory, certified by international safety standards
In November 2015, the FANCL B&H Nagano Factory received the "FSSC22000" international food safety standard certification. This sanitary factory environment for the safe production of germinated rice has been highly recognized by our business partners and the many customers who use our products. The FANCL B&H Mishima Factory also obtained the same certification in November 2022. With this certification, we will continue efforts to further enhance levels of food safety and product quality assurance, and ensure that customers in Japan and overseas can use our products with peace of mind.


Supplier inspections and training
In addition to Group companies, we also carry out inspections of suppliers to which we outsource manufacturing. In principle, these inspections are carried out once a year for cosmetics products and as frequently as necessary for other products.
These inspections cover the effectiveness of quality systems based on GMP※ and other standards, the state of manufacturing and quality controls, the state of facility and equipment management, and the state of employee education. They also ensure that necessary corrections and improvements are being made and that suppliers are aware of and responding to changes in regulations when required.
We also carry out information sharing and exchanges of opinion to enhance the knowledge of our Group companies and suppliers.
Good Manufacturing Practice: Standards for production management and quality control.
Thorough quality controls to ensure safety and reliability
We have built an inspection framework for receiving materials that takes each material's track record of usage into account, as well as a double inspection framework for products in which they are inspected during processes involving each production robot and then by a quality control department. These inspections determine whether a product will be shipped.
- For materials, we check the test results report supplied by the manufacturer. Once the material is inhouse, we reinspect it to check appearance and condition, including confirming there has been no contamination, and also conduct a microorganism inspection and a constituent analysis. We also practice expiration date management to ensure we do not use any materials that have deteriorated in terms of quality.
- Products are inspected during manufacturing processes so we can detect abnormalities early and make corrections to the relevant process. If an abnormality is detected in a certain process, we do not allow the affected products to reach the next production process.
- The inspection process of our quality control departments cover both the content of a product and its final form. It includes an inspection of appearance and condition, a microorganism inspection, and a check for contamination. We have established a framework where the shipment of products that deviate from standards is automatically halted.
Improving the safety of health foods: Manufacturing controls that incorporate food defense
We are working to prevent the intentional contamination of food products by establishing and constantly enhancing food defense systems. This includes introducing biometrics to limit access to manufacturing areas, installing cameras in manufacturing areas, requiring specified clothes to be worn, and controlling items that are allowed to be brought in.
- Only specified personnel are allowed to enter our factories. Furthermore, some of our factories use biometrics to control and record who enters production zones.
- We have installed cameras within factories so we can confirm the movement of people and products.
- We have made rules that limit the items that can be brought into production sites.
- Poisonous substances are kept locked up and thoroughly managed, and this management is carried out by a limited number of people.
- We provide employee education to raise awareness regarding food defense.
A support system that ensures abnormalities are not missed after sale
The mission of our specialist staff is to provide customers with beauty and health support, so they stay familiar with customer concerns regarding cosmetics, supplements, and foods.

- Feedback from customers is shared inhouse
- Evaluations of reports concerning changes to skin
If we receive a report about a change to skin, we appoint a safety and quality supervisor, who has expert knowledge concerning cosmetics safety, to evaluate and investigate if there is a connection to our products
- Evaluations of reports concerning changes in physical condition
If we receive a report about a change in physical condition, we appoint one of our expert employees, which includes medical practitioners, to evaluate and investigate if there is a connection to our products
- We carry out regular product sampling inspections
